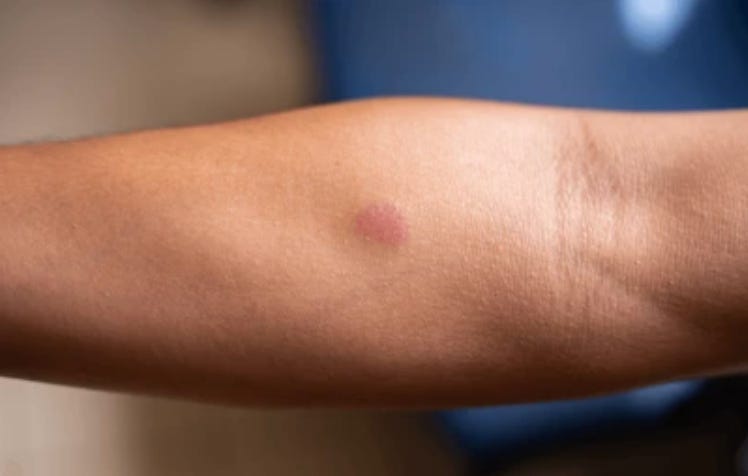
A couple of days ago—thirteen years after my first diagnosis—I completed my isoniazid regimen. 🍾
(If you’re a new subscriber, welcome! This is a continuation of my journey with isoniazid, which begins here.)
TB has followed me from the beginning—at least, the beginning of my word-that-is-not journey as a lab rat. I have no idea where I might have been exposed; only that I failed my first screening for TB, at a CRO by my old house in Austin, meaning that I was almost never a lab rat at all.
The test this CRO used was the infamous Mantoux test—or tuberculosis skin test. Developed by German physician Felix Mandel in 1908, and based on Robert Koch’s discovery of tuberculin, the Mantoux test works by injecting 5 tuberculin units into the left forearm. (Why the left? Can anybody tell me?) Then you wait 48 to 72 hours to see how much the skin has raised. There is a tool called a caliper (it looks like an IKEA wrench, but made of cardboard) which is used to measure the diameter of the reaction, along with a general guide (included below—though I should mention that everyone’s is different). Anyway, once I returned in 48 hours and my reaction was measured, the diameter was one millimeter too big for this CRO’s eligibility criteria—and just like that, I was given an immediate and life-long ban. The only way back in was to complete treatment with my local infectious disease program.

Of course, to be told you failed a test for TB is worrying, and I freaked out. I was twenty-four. I didn’t have health insurance. Asking around for cheap or free services, a friend gave me the address of an aging hippie doctor who accepted sliding scale cash payments. I paid $75 for a visit, $75 for a chest x-ray, and within 15 minutes I was out of there with a paper that said, ‘No evidence of TB disease.’ But that still wasn’t enough to get reinstated. The Austin CRO insisted that I receive TB treatment in order to be eligible. I figured it was a fluke—a bad reading—and resigned myself to screen for out-of-town studies.
This was 2012. For the ten years I lived in Austin, TX, I never did a study at the CRO that was down the street.
There aren’t many Phase I healthy clinics in the U.S., but a decent amount of them are in Texas—at least four, to my knowledge. The CRO in Austin is one of the few that screens for tuberculosis as a part of its prescreening process, a move that could be seen as either benevolent or malignant: a public health safeguard or a fail-safe for a clinical trial population that increasingly comes from places where tuberculosis is far from controlled. Another is a CRO in Dallas. (Not for all studies, but some.) And it was from them, eleven years later, that I received a call telling me I’d failed their screening* because my blood had tested positive for tuberculosis.
*The drug was called peresolimab. (It’s still in trials.) Developed by Eli Lilly and Co., peresolimab wasn’t linked to any particular conditions on its consent form, but rather the very general “autoimmune and inflammatory diseases.” The consent form made a point to mention it hadn’t been approved by the FDA to treat these conditions, but it had been approved for research. The risk profile was thorough—a surprising but welcome first for consent forms. This is how I learned that because it affects the immune system, peresolimab could make you more susceptible to developing infections and more prone to contracting viruses, and it could make vaccines less effective. Hence the TB screen.
“You should get it checked out,” a recruiter told me politely on the phone, “and unfortunately you’re disqualified for any future studies until you do.”
He said I was either exposed to tuberculosis or had latent TB—either way, I would remain banned until I completed treatment with my local infectious disease program.
This time, I had health insurance. After confirming the diagnosis with my doctor and the state tuberculosis clinic (latent TB), and weighing the pros and cons of treatment, I—normally a subject—became a patient.
The first thing I did after finishing my pills was get good and drunk. Isoniazid is notoriously contraindicative to alcohol, threatening to cause jaundice and other nasty things if mixed with the pills. (It was actually one of the first prescription antidepressants—terminal TB patients at a sanatorium in Staten Island who took it in an early trial were noted to be “dancing in the halls tho' they had holes in their lungs”—but was quickly pulled after it was found to cause liver toxicity.)
Other things to avoid with isoniazid include aged cheese, cured meats, fava beans, sauerkraut, soy sauce, beer, red wine, skipjack, tuna, mackerel, and salmon, which can cause symptoms like headache, sweating, flushing, palpitations, dizziness, lightheadedness, or feeling faint. These are some of my favorite foods, and it’s safe to say I did not follow this instruction (and paid for it).
The next thing I did was call the Austin CRO, hoping to be reinstated. The recruiter on the other end of the line had some bad news. Last October, the board had decided that all positive TB tests were exclusionary for their healthy studies. Guess I would never do a study there.
So I called the CRO in Dallas.
They told me it had been a while since I had updated my profile and I had to update my health history. “Do you have any metabolic conditions?” the recruiter asked.
No, I said. I wasn’t sure what she meant—but it didn’t matter. No was the way forward.
And then: Do you have any stomach or colon conditions? Have you had your gallbladder removed? Do you have a history of hair, eyes, ears, nose, or throat conditions? Have you given or received a blood transfusion? Do you have any neurological or psychological conditions? Do you have any conditions involving your lungs? In the last three months, have you taken any prescription medicine? Any illicit drugs or tobacco use? etc.
The study I was interested in was being developed as a potential treatment for autoimmune diseases. That’s all I usually knew before coming in for a screening: a vague idea of what the drug was supposed to do—if it worked. I didn’t know what kind of autoimmune diseases the investigational drug was developed to treat, or how exactly the investigational drug was supposed to act in my system. Usually, the recruiter on the phone line knew as little as me. There were often a dozen or more studies occurring at any time. How could she even keep track of them all?
Screening for studies was an exercise in double consciousness. On the surface, you had to make sure that you were the most eligible. That you were calling early enough to get into one of the first screening appointments—especially if you were traveling a long distance, as I was—and getting paid well for your time. That was the primary goal in a Phase I study. There were 22 people max in a cohort. Cohorts filled quickly, and CROs always recruited 4-5 backups. That meant you could realistically drive to a screening, pass, then return for the actual trial, only to be sent home with $50. ($250 if they asked you to spend the night.) This was what you always wanted to avoid. (Of course, you also asked yourself: Will I be safe? But this often wasn’t in sync with ‘Is this the best one?’)
What recruiters wanted to avoid was going through the barrage of questions, only to have an aspiring subject back out at the last minute. To remedy this, they usually led with the study’s shittiest aspect; its ugliest angle. So after I checked and double-checked the screening schedule, ensuring that I would have a spot in the first group, the recruiter asked me, as an opener, “Will you be OK with a skin sample?”
“Could you tell me more about that?” I asked, because I was taken aback.
It was a patch, that’s all she knew, that sliced a little bit of your skin. The amount taken would be the size of a quarter. It would be taken from the back, she said. Or maybe the torso. Processing this new information, my on-the-fly research led me to skin biopsy. This was the technical term for the procedure (No wonder she didn’t use it, I thought) of which there were three different kinds, a website told me:
A shave biopsy removes a sample from the top layers of skin with a razor blade or scalpel (a small cutting blade used for surgery). Your provider will do a shave biopsy if your condition appears to involve only the top layers of skin.
A punch biopsy uses a special tool with a round blade to remove the skin sample. Your provider will do a punch biopsy if your condition appears to involve the deep layers of skin.
An excisional biopsy uses a scalpel to remove all of the skin lesion, usually with some normal skin around it. The sample may include the full thickness of the skin along with fat below the skin.
“You may need to have your body cut or shaved if it interferes with the patch,” she said.
“OK,” I said. More new information.
This was the study conundrum at work: I could have done more research. Said I’d call back. But there was no guarantee the study would be there when I called back. Or any study, for that matter.
So I said yes.
And then she asked me a question that made me more uncomfortable.
“Are you OK if we obtain and store a blood and skin sample for future research?”
More new information. And I wasn’t OK with it—not without more information. (Who was the sponsor? How would they use my blood and skin? These were my major concerns.) What’s more, this felt like a whole new thing to agree to. I’d already agreed to take the drug, and give a bit of my skin, and now I was agreeing to let a sample of my skin and blood be obtained and stored—full stop.
I said yes.
Not missing a beat, the recruiter followed up with some study-specific questions. Any allergies to food, medications, anything? Any history of psychological problems, depression, or suicidal thoughts? Any history of thyroid disease? Have you received or intend to get any vaccines within 28 days prior to check in? Any surgical procedures in the past 12 weeks? Any history of cancer? Any treatment or hospitalization in the past 3 months? Any history of herpes in the last three months? Any history of tuberculosis?
“Yes,” I said, as relief poured over me. “I have latent tuberculosis.” I told her the spiel the state tuberculosis department had told me. I’d completed treatment, but I’d always test positive for tuberculosis.
“Oh,” she said. “I see it now in your file.”
She put me on hold.
“I’m sorry,” she said when she returned. “A history of active or latent tuberculosis is an exclusion. Do you want me to help you find a study where TB isn’t excluded?”
I thought that completing my isoniazid treatment would open me up to a whole new world of studies—but instead it seems to have followed me around, even post-treatment.
I want to say something that I think is true. Despite (or maybe because of) my setbacks, I sometimes feel the greatest benefit of my guinea pigging career has been my circuitous, and practically accidental, medical education. This is an education I’ve gained through ineligibility, and it never meant I had the means to treat my ailments—quite the opposite. But as anyone who’s dealt with sickness can attest (i.e., everyone), health knowledge is a kind of power, even if it’s limited by our means (money, resources, time). And I do feel as if that knowledge is empowering, even if I want to push back on self-advocacy as an ideal.
In The Social Transformation of American Medicine, historian Paul Starr’s magnum opus of healthcare in the United States, Starr tracks how, starting in the Reagan years, healthcare plans became more varied, while requiring more buy-in from the consumer. EPO, HMO, POS, and PPO plans each offered unique perks. (Even if you always had to sacrifice something else.) “Choosing” your plan was a kind of advocacy: your specialized path to healthcare. As health insurance costs shifted from employers to workers in the early 2000s, leaving many underinsured, our very terms changed, reflecting this new way of looking at health, one I’m not sure we’ve moved past: Primary Care Physicians became “patient advocates.” Patients who advocated for themselves became “empowered.” (Human research subjects, I would say, became “entrepreneurs.”)
Choice!—the neoliberal death knell. And yet: educating myself on my own health, and writing about my journey to find healing, has been me choosing to see myself as a person who is capable of healing. And in a culture where the death drive is so strong, I wish that on everyone.
I’m in the latest issue of Brevity, out today. Here’s a link if you want to read. Brevity is one of my favorite magazines and to see myself in it is… dreamy.

Hope y’all have a good week. Thanks for reading,
—Cory the Rat